Our Mission
Clinically
Tested
Regenerative Capabilities
Unparalleled
Patient Outcomes
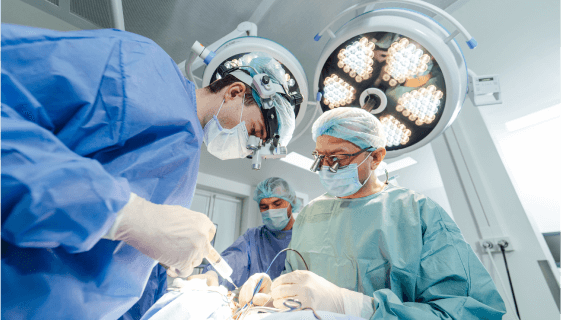
Dermacyte Matrix:A Cutting Edge Approach for Advanced Wound Care
Dermacyte® Amniotic Membrane Matrix (Dermacyte Matrix) is a human amniotic membrane allograft with a preserved epithelial basement membrane and compact fibroblast layer.
Our product is a commercially available extracellular tissue product and is intended for use as a protective covering for partial and full thickness wounds and surgical sites.
Based on proprietary advancements in amniotic tissue processing, Dermacyte Matrix is created from amniotic tissue that undergoes rigorous screening and is produced under the highest quality standards.
A Powerful Regenerative Wound Matrix to Support Acute and Chronic Wound Closure
Dermacyte® Amniotic Membrane Matrix (AMM) is derived from human postpartum amniotic tissues that have been processed to retain native extracellular matrix cytokines, growth factors, and collagen to support tissue remolding, improving patient outcomes and treatment comfort.
Dermacyte AMM contains:
- Anti-inflammatory cytokines, growth factors, and other molecules that help to reduce inflammation in damaged tissues.
- Anti-scarring properties to reduce scarring in wounds and promote the formation of healthy, functional tissue.
- Anti-microbial molecules that help fight off microbial infections.
- AMM has been shown to modulate the immune Response, reducing inflammation, and promoting tissue repair.
Dermacyte AMM also offers a number of other biological functions that make it attractive for use in clinical applications. These include:
-
Promotion of angiogenesis: Dermacyte contains molecules that can promote the formation of new blood vessels, which is important for tissue repair and regeneration.
-
Provision of a scaffold for cell growth: The stromal layer of a amniotic membrane provides a scaffold for the growth of cells, making it useful for tissue engineering and regenerative medicine applications.
Unparalleled benefits
Protection
Versatility
Clinically Tested
Storage Efficiency

Why is wound care essential?
- Globally, 50 million (9M in US) people suffer from chronic wounds.
- Chronic wounds impact the quality of life of nearly 2.5% of the total population of the United States.
- Given the aging population, the continued threat of diabetes and obesity worldwide, and the persistent problem of infection, it is expected that chronic wounds will continue to be a substantial clinical, social, and economic challenge.4
Dermacyte Matrix is regulated by the U.S. Food and Drug Administration (FDA) as a minimally manipulated human allograft tissue under its Human Cells, Tissues, and Tissue-Based Products (HCT/P) guidelines, subject to Section 361 of the Public Health Service Act and 21 CFR 1271.
Our products are the proven choice in the market


Supporting better outcomes in wound care
Learn why leading healthcare professionals prefer to use the Dermacyte Matrix for advanced wound care
Learn why qualified patients request using the Dermacyte Matrix for advanced wound care.
Antimicrobial Technologies LLC